 0
0
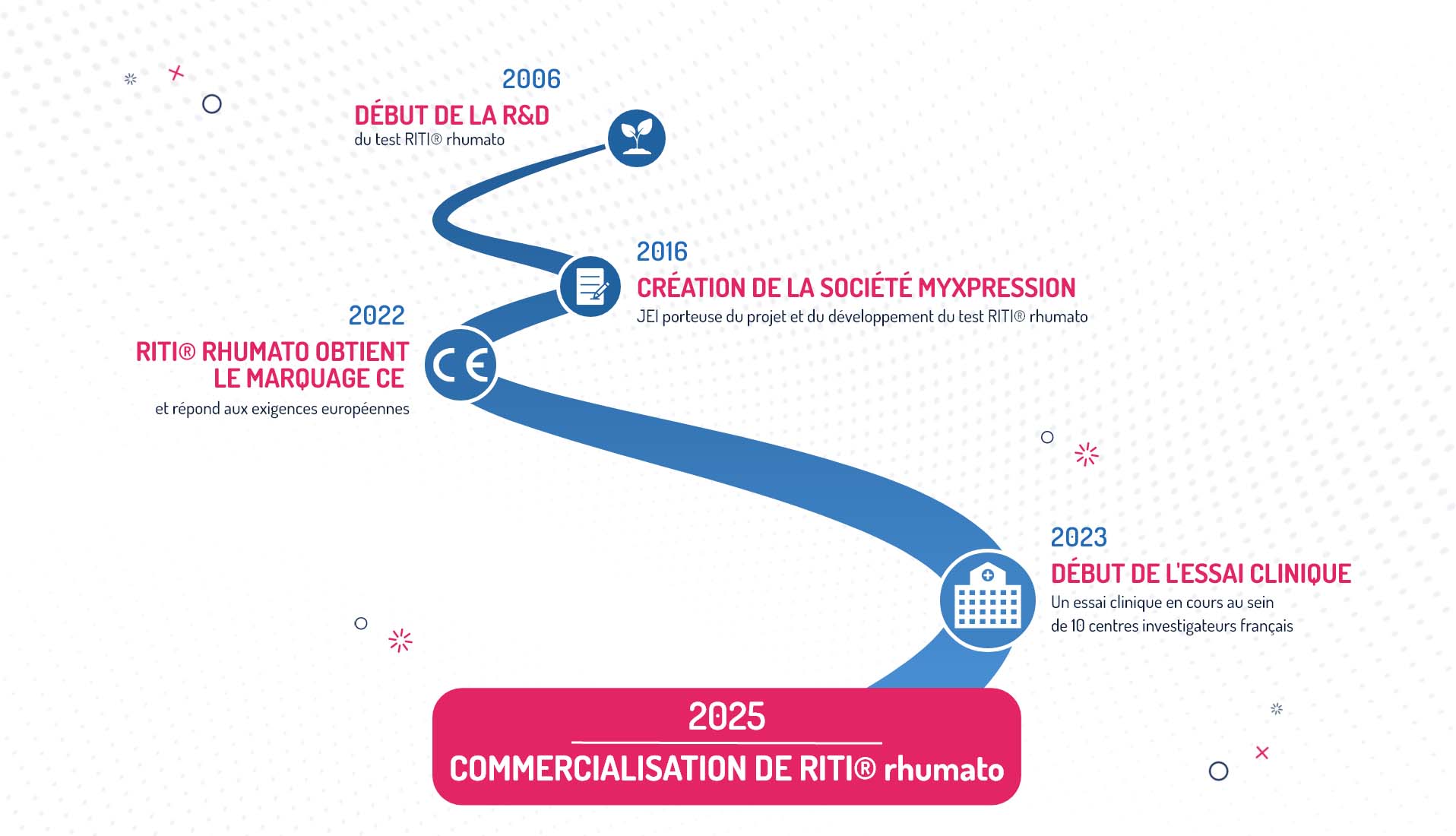
le marquage CE
au sein de 10 centres investigateurs français
rhumato




en remplissant le formulaire ci-dessous* :

F.A.Q
Lorsque le test RITI® rhumato sera commercialisé, une interface médecin/patient sera mise à votre disposition afin que vous puissiez gérer les prescriptions et les résultats des tests de vos patients.
Pour disposer du test RITI® rhumato, il vous faudra faire une demande auprès de votre médecin rhumatologue qui souhaite vous prescrire une biothérapie. Vous pouvez lui présenter la solution RITI® rhumato en téléchargeant le document « en parler à mon médecin rhumatologue »
Il est déconseillé de vous procurer le test RITI® rhumato sans en discuter au préalable avec votre médecin rhumatologue référent; En effet, seul lui est habilité à vous prescrire une biothérapie et donc à choisir ou non d’exploiter les résultats de votre test pour faire son choix.



