A clinical trial is in progress to demonstrate the efficacy of RITI® RA. The trial aims to show the relevance of the RITI® RA test when treating rheumatoid arthritis. The final main stage before the marketing of the innovation is currently planned for 2025.
The clinical trial in figures
- 12 university or general hospital centers spread throughout France
- 234 patients included
- 12 months of study
Stay informed by completing the form:

Rheumatoid arthritis: which biologic therapy to prescribe for my patient?
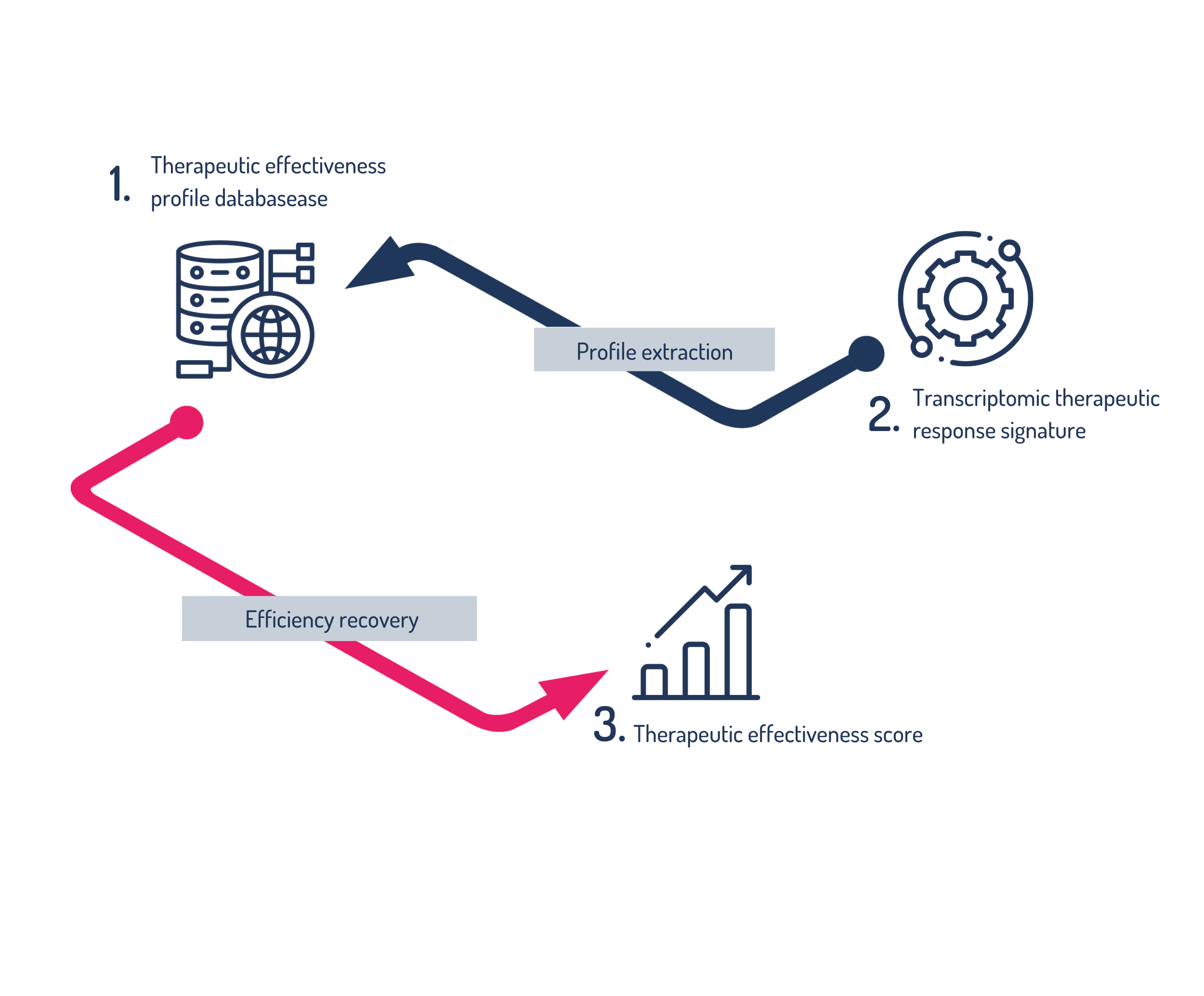
By comparing its database of efficacy profiles and your patient's transcriptomic signature , the RITI® RA test can calculate Therapeutic Efficacy Scores for each biologic therapy.
How does it work?
Step 1
Collection of a blood sample from the patient.

Step 2
You access your patient's report, where you find the efficacy score for each biologic therapy. You can then make an informed choice regarding the therapeutic strategy to adopt for your patient.

Step 3
The dedicated algorithm performs billions of calculations to determine, for each biologic therapy, their efficacy score for the patient.

Results
You access a personalized report, in which you find the efficacy score for each biologic therapy. You can then make an informed choice regarding the therapeutic strategy to adopt for your patient.


Ten years of R&D were required to build a unique database based on international clinical studies.
After identifying the most relevant studies for rheumatoid arthritis, raw data was extracted and stored to be transformed into high-value information. From this unique expertise, a database was created, consisting of elements of immune dysregulation specific to rheumatoid arthritis, supplemented with information specific to each existing biologic therapy. This database is used to establish the patient's efficacy score for different biologic therapies.
Upcoming Market Launch
The RITI® RA test is set to be commercialized in 2025. Prior to the market launch, mYXpression Rheumatoid seeks your input to provide essential information for using the technology. Feedback is anonymous and remains confidential. To do so, we invite you to share your suggestions, comments, or questions regarding the RITI® RA test below: